bipolar disorderschizophrenia
IGALMI is proven to help reduce agitation
Limitations of Use: The safety and effectiveness of IGALMI have not been established beyond 24 hours from the first dose.
Can help you feel less agitated
May start working in as early as 20 minutes20 to 30 minutes†
IMPORTANT SAFETY INFORMATION
IGALMI can cause serious side effects, including:- Decreased blood pressure, low blood pressure upon standing, and slower than normal heart rate, which may be more likely in patients with low blood volume, diabetes, chronic high blood pressure, and older patients. IGALMI is taken under the supervision of a healthcare provider who will monitor your vital signs (like blood pressure and heart rate) and alertness after you take IGALMI to prevent you from falling or fainting. Make sure you are adequately hydrated and sit or lie down after taking IGALMI. Tell your healthcare provider if you feel dizzy, lightheaded, or faint.
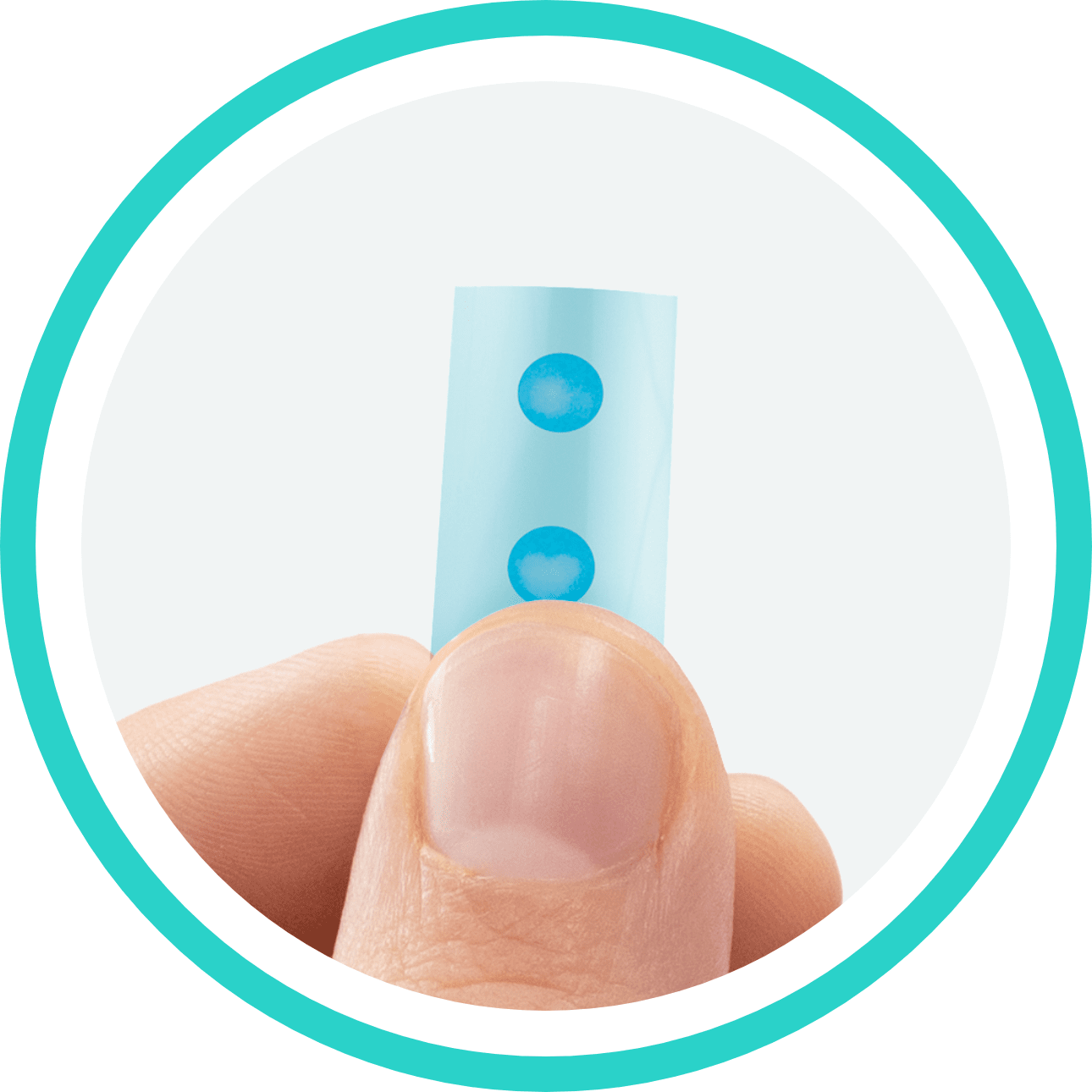
WHAT IS IGALMI?
IGALMI® (dexmedetomidine) sublingual film is a prescription medicine that is placed under your tongue or behind your lower lip and is used for the acute treatment of agitation associated with bipolar disorder I or IIschizophrenia in adults. The safety and effectiveness of IGALMI has not been studied beyond 24 hours from the first dose. It is not known if IGALMI is safe and effective in children.
- Heart rhythm changes (QT interval prolongation). You should not take IGALMI if you have an abnormal heart rhythm, a history of an irregular heartbeat, slow heart rate, low potassium, low magnesium, and if you are taking other drugs that affect your heart rhythm. Taking IGALMI if you have a history of abnormal heart rhythm can increase the risk of torsades de pointes and sudden death. Tell your healthcare provider immediately if you feel faint or have heart palpitations.
- Sleepiness/drowsiness. Do not do activities requiring mental alertness, such as driving or operating hazardous machinery, for at least 8 hours after taking IGALMI.
- Withdrawal reactions, tolerance, and decreased response/efficacy. IGALMI was not studied for longer than 24 hours after the first dose. Physical dependence, withdrawal symptoms (e.g., nausea, vomiting, agitation), and decreased response to IGALMI may occur if IGALMI is used longer than 24 hours.
The most common side effects of IGALMI in clinical studies were sleepiness or drowsiness, a prickling or tingling sensation or numbness of the mouth, dizziness, dry mouth, low blood pressure, and low blood pressure upon standing.
These are not all the possible side effects of IGALMI. Speak with your healthcare provider for medical advice about side effects.
Tell your healthcare provider about your medical history, including if you suffer from any known heart problems, low potassium, low magnesium, low blood pressure, low heart rate, diabetes, high blood pressure, history of fainting, or liver impairment. Tell your healthcare provider if you are pregnant or breastfeeding or take any medicines, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take any drugs that lower your blood pressure, change your heart rate, or take anesthetics, sedatives, hypnotics, and opioids.You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1‑800‑FDA‑1088. You can also contact BioXcel Therapeutics, Inc. at 1-833-201-1088 or medinfo@bioxceltherapeutics.com.
Please see full Prescribing Information.
WHAT IS IGALMI?
IGALMI® (dexmedetomidine) sublingual film is a prescription medicine that is placed under your tongue or behind your lower lip and is used for the acute treatment of agitation associated with bipolar disorder I or IIschizophrenia in adults. The safety and effectiveness of IGALMI has not been studied beyond 24 hours from the first dose. It is not known if IGALMI is safe and effective in children.